Hey Steemians, Welcome again to my blog.
Two days ago, I wrote about corrosion but focused more on the cost implications of corrosion. However, due to feedback from my followers I will be discussing the chemistry governing corrosion and we will proceed into the adverse effects of corrosion as well as how we can prevent corrosion. Having said this and without wasting time, let’s get down to business.

INTRODUCTION
Let me start by defining corrosion:
Corrosion is an unwanted deterioration or depletion of a material arising from its interaction with its environment. The environment here is any region within the vicinity of the material. This could be gaseous, liquid or a molten solid. I want you to note that corrosion is a material removing process and it is usually electrochemical. That is, usually corrosion are chemical reactions that involves the transfer of electron between chemical species.
But does that mean corrosion occurs only in metals? No, corrosion occurs in other types of material. However, the corrosion of non-metals is usually solely chemical. An instance of non-metal corrosion is the corrosion of glass in the presence of superheated water. You can check out this link to find out more.
Since metals take a substantial proportion in the material we utilize, especially in industries, the corrosion of metals is obvious. Consequently, we will look at the mechanism governing the corrosion of metals. Nonetheless, have it at the back of your mind that the underlying concept of corrosion is that it is a material removing process and this applies in all cases.
MEHCANISM OF METAL CORROSION
I have noted earlier that metallic corrosion is normally electrochemical and from elementary chemistry, this is a chemical reaction involving the transfer of electrons. One chemical species losses electron (usually the metal) and the other species acquires the lost electron (isn’t life not just all about give and take?).
The chemical species which gave up the electron(s) becomes oxidized and the other which gained this electron(s) is reduced. The former is called the Reducing agent and the latter is the Oxidizing agent.
Come to think of it, what does metal oxidation has to do with corrosion? The answer to this in not lengthy – Metal oxidation is metal corrosion (Yes, it is as simple as that). You can also call in metal dissolution as we will see soon.
Let’s introduce oxidation equation.
Just picture a Hypothetical metal in your mind. This metal could be any of the existing metals, but let’s denote it with “N”. We shall denote its valency by “n”. The Oxidation equation involving this metal is written as follow:
We can see from above that N losses n number of electrons and becomes a positive ion. That is, N has been oxidized. This is the basics of corrosion.
But don’t you think we are missing something?
Of course, every electrochemical reactions has two half-reactions. The oxidation half-reaction and the reduction half-reaction. Both comes together and occurs simultaneously to give a complete electrochemical reaction. To introduce the reduction half-reaction, let’s consider a real example. The corrosion of solid Zinc in a concentrated acid solution which is rich in hydrogen ion is shown.
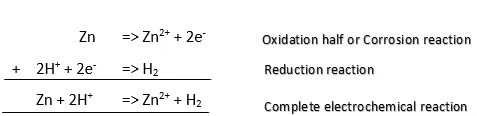
Image created by me (@temitayo-pelumi).
Zinc has a valency of 2 and losses 2 electrons which is acquired by 2 hydrogen ions to give one molecule of hydrogen gas. From the oxidation half-reaction, you can see that Zn becomes oxidized to Zn2+ i.e. Zinc is dissolved. One point to note in these is that Oxidation reaction is the actual corrosion process but will always be accompanied by reduction reaction.
Before we move to the next section, I like to clarify a misconception.
Many people take the word Rust to mean Corrosion. However, let me state clearly that Rust is only a Kind of corrosion – the corrosion of ferrous metals (i.e. metals containing Iron). Corrosion on the other hand is an umbrella term.
ADVERSE EFFECTS OF CORROSION
In my previous post on corrosion here, I discussed extensively, the monetary losses that stems from corrosion. Due to this, I will focus on other kind of losses arising from corrosion. The following are some of the adverse effects of corrosion.
- Plant Shutdown
There should be no doubt about the inclusion of this in the list since corrosion leads to the failure of plant equipment and facilities. The failure of such resources may result in the complete or partial shutdown of the plant.
Let me point out a case during my industrial training in a power generating plant. There are six generating units in the whole plant with each producing 220 MW of power. We had to shutdown a complete unit just because of a tube failure in a feedwater heater. There are six feedwater heaters per unit and the failure of just one resulted in bringing down a whole unit (well, feedwater heaters are that important).
It took about three days to restore the heater to normal condition. What do you think I’m trying to point out? Imagine what could have been generated within such period. I think you get the drift now.
- Tarnishing of Material Appearance
If you are someone like me who likes to attend to faulty stuffs in the house, then you should be able to relate here. But let’s do a simple task to get a better view.
Take a sheathed copper wire. Peel off the sheathing to expose some portion of the copper wire. Leave the wire for a day and check it on the next day.
Really? Will you have to come back to finish your reading tomorrow? Of course not, you should save the task for later as I will give you the answer. Here is what I want you to observe from the task.
The exposed copper wire should be initially bright when you peel it and when you check on the next day it will appear dull. You can even peel more portion this time to compare and see the difference.
Having said this, it is obvious that corrosion tarnishes the appearance of a material and leaves bad impression on people since corroded things are not pleasing to the eye. I can share a personal story on this, but I don’t want to make the article lengthy.
- Contamination of Products
Do you wonder how this is possible? Here is how.
Remember I noted that corrosion involves dissolution of a material to form ions. Imagine if such metal ion mixes with an unfinished product during production, isn’t that contamination?
When a product is contaminated, its quality is adversely affected and its market value becomes poor. Such is unwanted in any production setting especially in a food processing or pharmaceutical industry. Products from such industries require a high degree of purity as they are directly consumed by humans.
- Loss of Products
Corrosion can result in the loss of products as a result of leakage caused by corrosion. Example is the leakage of public water piping as a result of rust. In another case, the product could be of high value like crude oil or might be toxic such that when released into the environment, it poses threat on the ecosystem.
- Effect on Reliability and Safety
The reliability of an equipment is the probability that such equipment will not fail under its working condition over a certain period of operation. However, since corrosion is capable of resulting in unexpected failure, it thus adversely affect the Reliability of an equipment. Moreover, failure of equipment and plant facilities could result in accident which could compromise the safety of personnel and my result in loss of life.
HOW DO WE PREVENT CORROSION
From the above said, we have seen the nature of corrosion and what it costs us. However, we can minimize the costs of corrosion by doing the following.
- Material Selection
In this context, the key word for you to hold is “Compatibility”. To fabricate a product, there are countless number of materials to choose from. The principle of material selection is choosing a material that will perform well against corrosion for the intended application. Let’s see an example.
Buglarproof frames installed on windows are usually fabricated from mild steel. Mild steel is rich in Iron which makes it corrode in a moist environment which is where the burglarproof is used (Air contains moisture and oxygen which attacks iron). Buglarproof frames are usually painted to protect them from corrosion.
In another case, sliding window frames are made with Aluminium. This aluminium window frames are in the same vicinity as the burglarproof frames (that is, they share the same environment). However, Aluminium does not require painting because it does not corrode in such atmosphere i.e. it is compatible.
I believe this example gives you a better understanding of the concept of “Material Selection”. Thus, when you want to utilize any material, ask yourself – is the material compatible with the environment of the intended application.
- Alteration of the Environment
Another way to prevent corrosion is to alter the environment wherein the material will be used. We can reduce some agents of corrosion such as pressure, temperature or concentration of the environment. High temperature and pressures are known to increase corrosion rate.
Alteration of the environment can be achieve by the use of inhibitors. These are substance which decreases the corrosiveness of an environment. Corrosion inhibitors utilizes different kinds of mechanism depending on the type employed.
For instance in the power plant where I had my industrial training, we dose hydrazine (N2H4) into the feedwater to remove dissolved oxygen in bid of preventing corrosion of plant facilities like the boiler tubes and turbine blades. The chemical equation governing this is given below:
- Cathodic Protection
Remember the oxidation equation of the hypothetical metal N we discussed at the beginning of this Article. Let me re-write it.
Recall that it involves the loss of electron by N, which then becomes an ion (Nn+) and free to go into solution (i.e. corrode).
What will happen if we supply electron to this metal?
Here is what will happen – the metal will want to keep oxidizing but the supply of electrons to it will annul the oxidation process. It is as simple as that.
Imagine you have a fully inflated sealed balloon and you make two pin-holes into it at opposite ends. If you can inflate the balloon on one side at the same rate it is deflating on the other side, then the size of the balloon will remain unchanged. This is similar to what cathodic protection is doing.
You should see this post by @rharphelle. He discussed extensively on cathodic protection.
- Designing Against Corrosion
I like to play the game of chess, although I wouldn’t say I’m a pro, but I’m not that bad. One secret in winning a chess game is to plan ahead for your future moves. Some professionals are able to plan more than four moves ahead. They will read the game and would have taken a lot into account. This is exactly what designing against corrosion looks like.
Taking corrosion into account during the design phase of a product is an effective means to combat corrosion. For instance, the design engineers could increase the wall thickness of a product to compensate for corrosion, since corrosion is a material removing process.
CONCLUSION
Corrosion is a pain in the ass as it is one major problem faced by many industries. It deprives us in many ways. However, we can minimize its adverse effects by good protection practices.
Thanks for reading through. I hope to have you here again.
REFERENCES
- Mars, G. F. (1987). Corrosion Engineering (Third Edition). McGraw-Hill Book Company.
- Callister, W. D. and Rethwisch, D. G. (2010) Materials Science and Engineering, An Introduction (Eighth Edition). John Willey & Son, Inc.
- Gerhardus, H. K., Michiel, P.H., Brongers, Neil, G. T., VIRMANI, Y. P. and Payer J. H. Corrosion Costs and Preventive Strategies in the United States. National Association of Corrosion Engineers. Publication No. FHWA-RD-01-156
- NACE study estimates global cost of corrosion at $2.5 trillion annually
- Corrosion of non-metallic materials (Part-2)



