Life is imperfect. So are we humans, residing and claiming ownership of everything the universe has gotten. As unfair as situation can be sometimes, or is it most times? Whatever, we rely on science and engineering to get us the best part of them. Well, truth is, even engineering can’t always get us everything we want.

Source: Pixabay (Public domain - CCO licensed)
Whether you are trying to put food into your belly or fuel into your car, you are always trying to get something out of raw materials. You are trying to convert energy. But if you want to talk about how this works, we need to talk about thermodynamics and the laws behind it. And then it can be truly understand how power energy is truly engineering.
Energy is constantly being converted all around you. When you take a bite of an apple, it takes in the food energy and converts it to something that your body can use. May be you use it to power your normal bodily functions or you might store the energy to be used later as fat.
So many energy conversions do not just happen on a personal scale, they also occur on many engineering designs, but with hydroelectric dam. In hydroelectric dam, water turns to turbine, which then turns to shaft in the electric generator, converting the movement of the water into electricity. This conversion is important, because energy doesn’t just come out of nowhere; it needs to come from other type of energy.
So, to better understand how energy can be converted, you need to understand thermodynamics. Thermodynamics is the branch of Physics and engineering that focuses on converting energy, often in the form of heat and work. It describes how thermal energy is converted to and from other forms of energy and also to work. And thermodynamics is one of the main focuses of mechanical engineering because thermal, as it is often called, is critical to engines.
Engineers need to know how much heat will work get in engines if they put energy into it. Even when the focus is on heat and engines or something, like heat pump in refrigerators, we still don’t want our machines overheating. After all, engineering is not just about getting what we want, but also controlling what we don’t want.
Get this right, it is not just only mechanical engineers that deal with thermodynamics, it also plays a big role in chemical engineering. When chemical reactions form new compounds, they often create energy. And often, that energy is thermal energy.
Zero law of Thermodynamics
Now, to understand how all this works, we need to start from the bottom – Zero law of thermodynamics. Yes, that is really what it is called. We only came to understand zero law of thermodynamic after his siblings, the first and second laws had already been established, but it was considered so fundamental to thermodynamics that it was promoted to be more than first. So, zero. Now, this whole focuses on temperature and defines thermo equilibrium.
Equilibrium
In general, equilibrium is where certain properties, like pressure, volume, or temperature remain the same across the system. So, if two or more systems are in equilibrium, then, they are all in the same temperature.
Zero law states that when two objects individually are thermo-equilibrium with the third object, then they are also in equilibrium with each other.
This is very important because when a body is left in the medium, and at a different temperature, energy will be transferred until thermo equilibrium is established. That is why when you leave a cold solder in the sun, you will come out with the same temperature as the air outside. The basic ideas behind why this happens are explained by the next law – the first law of thermodynamic.
First law of thermodynamic
The first law of thermodynamic supports the law of conservation which we discussed about [here]. The basic defines heat as a form of energy which means it can neither be created nor destroyed. So the can’t create or destroy energy but we can convert it from one form to another. This might seem pretty simple but it is a powerful idea. In order to better understand the system, how we can get energy from it or how we can store the conversion of energy, but we won’t.
No matter what system we are looking at, there are few areas of energy that we need to concern ourselves with. The energy contained within a system and the energy that can move between boundaries.
Kinetic energy
Let us start with the energy inside the system. We can break it down into three main parts. The first is kinetic energy – the type of energy involved with movement. Mostly come in form of translational kinetic energy, which is when something moves from one location to another.
There is also one called rotational kinetic energy – when something spins or rotates. And then vibrational kinetic energy – when something shakes or vibrates. Think about it in terms of playing a baseball. As it flies in the air, the ball has kinetic energy. The kinetic energy will be translational as it moves from your hand to the targeted destination, and rotational as it spins through the air.
Potential energy
The second type of energy inside the system is the potential energy – the energy that can come from where something is, even if it is not moving. We can basically think of this as a stored energy. Potential energy often has to do with how high something is. The higher it is, the more potential energy we can have. This often called gravitational potential energy. When you are climbing a ladder, you have more and more potential energy with every step you take.
Potential energy can also come from object in horizontal position. Think about a bow and arrow. Using elasticity, we can transfer our potential energy to the arrow as we draw it back through the bow. As we fire the arrow, potential energy is transformed into kinetic energy.
Internal energy
The third energy that we find in the system is a bit different. It is called internal energy. This is the energy associated with the seemingly random movement of molecules. It is similar to kinetic or potential energy but on a much smaller microscopic scale. Take a glass of water for example. As the glass sits down on a table, the water doesn’t seem to be moving. But on a microscopic level, the water is teaming with molecules that are travel ling around in super high speeds.
While the internal energy might not seem so important, it can have major effect on a system. That is because changes in internal energy can result in changes in temperature, changes in phase like from solid to gas, or changes in chemical structure. All these types of energy kinetic, potential or internal show us what can exist within a system. But these types of energy can cause a boundary from their system to the surroundings.
One of the main types of energy that can cross boundaries is heat, which is the flow of thermal energy, and another is work, which is essentially any type of energy other than heat. So then all of these different types of energy involved with a system can help us understand the first law of thermodynamics.
Let us talk about a closed system, where flow is moving in or out. A good example will be a piston in a closed cylinder.
The first law of thermodynamics states that change in internal energy, kinetic energy and potential energy of a system is equal to the heat added to the system, subtracted by the work done in a system.
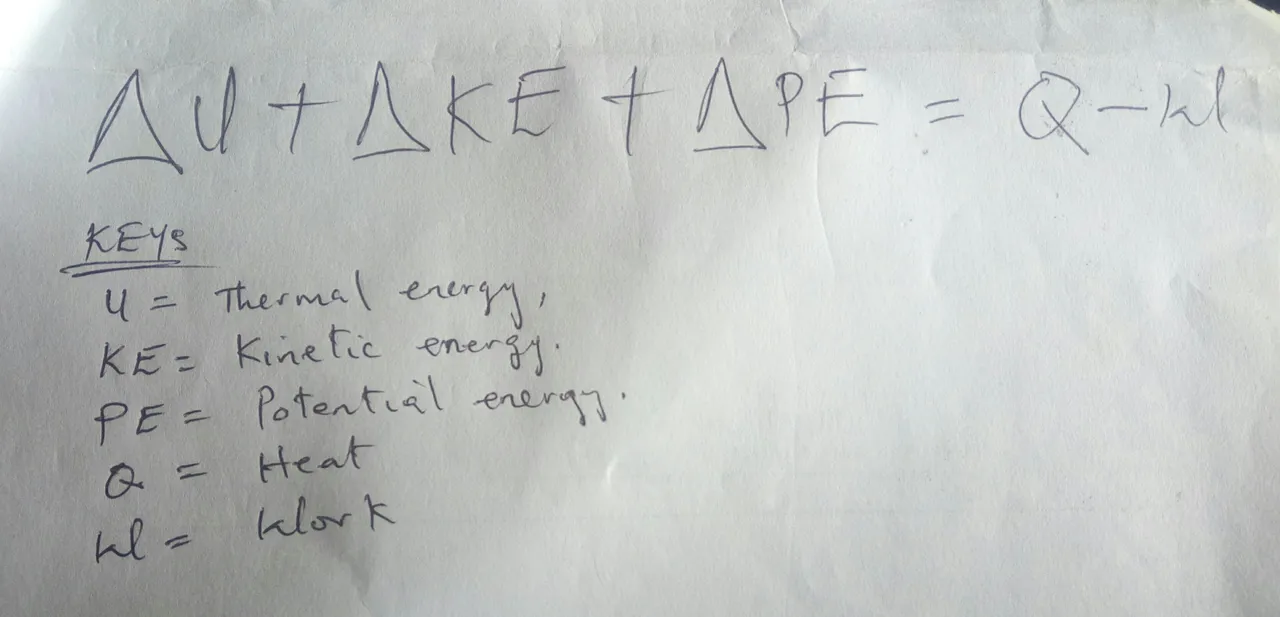
This may look a pretty complicated but there are a few scenarios that help us out. One is these is stationary system. If you look at the left side of the equation, you will see that a changes in kinetic and potential energy will be zeros for a system that is not moving making us have what we have in the below:
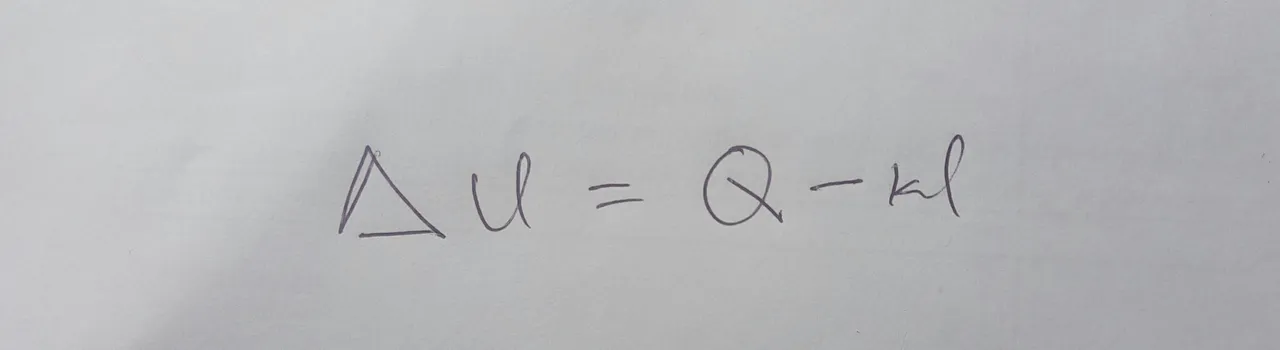
Another special case is in the adiabatic process. An adiabatic process is when there is no heat transfer, meaning not to be passed. This can happen if there are no different temperatures or something is so well intubated that only a negligible amount of heat can pass through the boundaries.
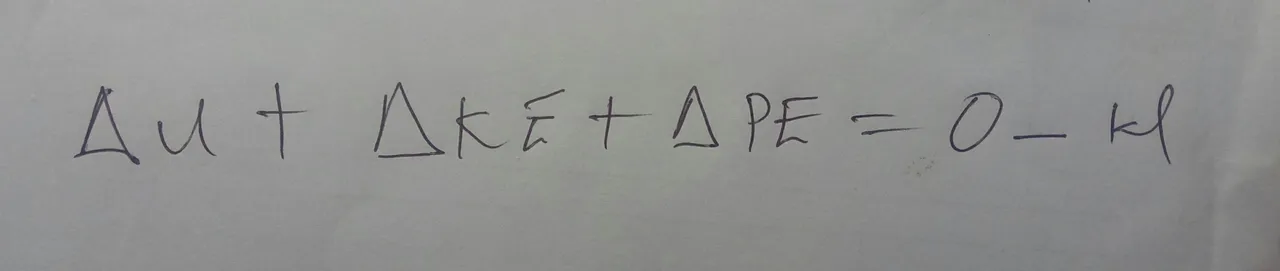
Think about like how a good thermos bottle can keep your hot chocolate warm. We can also simplify this equation if we can have an isochoric process. When a process is isochoric, the volume of the system remains constant. This often means that there won’t be any work, leaving us with only heat on the right side of the equation.
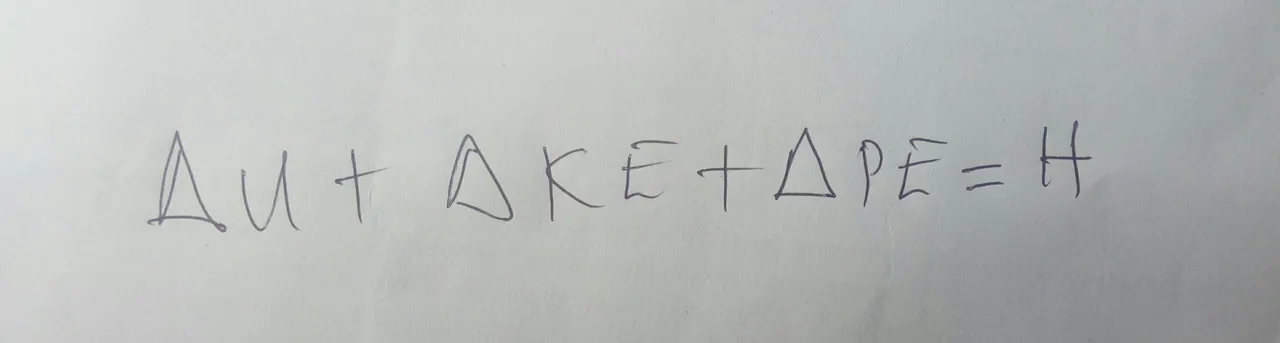
Any of these special cases helps give you a much simpler equation to work with, but this all has to do with a closed system. Often time, you will find yourself dealing with more complex open systems. Unlike closed systems, open systems have a flow going in and out. A good example will be if your basement flooded and you need to pump the water out of it. With a system like this, you will need to introduce a different measurement – entropy.
Entropy includes internal energy, but also adds in the energy required to give a system its volume and pressure. For an open system, you will also want to refine what you mean by work. Here, you want to focus on shaft work, which is basically, any type of mechanical energy other than what is necessary for flow. Going back to our equation, you will want to replace your internal energy with entropy and change the general work to focus specifically on shaft work. This will apply your law to open systems as well.
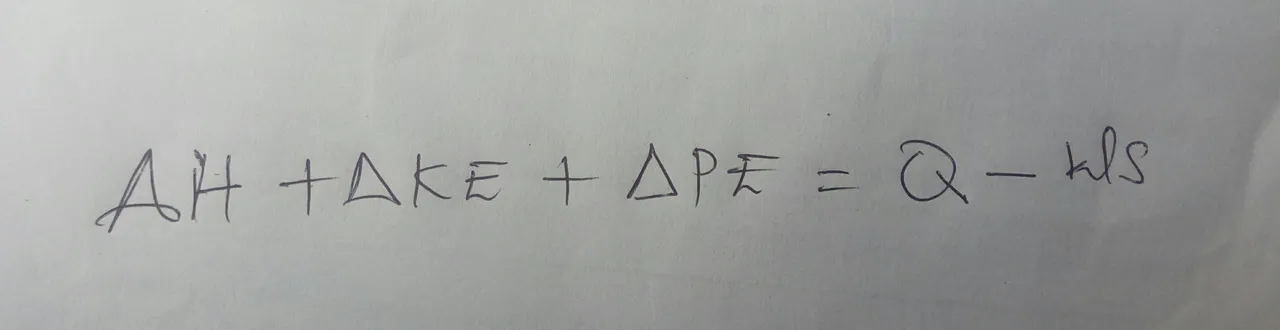
Let us use a flooded basement as our open system. First of all, we should establish that what we treat in the basement is our system and the outside, where we want the water to go, is our surroundings. When we run the pump, it will take in electricity and convert it to shaft work, which turns the pump. That energy will then be used to get the water moving which will change some of its potential energy to kinetic energy.
Hydroelectric dam is an open system too. If you think of the dam as a system and its environment as its surroundings, then you see that there is flow coming in in form of water, and flow coming out in form of electricity.
Final thoughts
It is a little more complex than just draining your basement and it will take a lot longer to learn everything involved in generating electricity, but the laws behind it are exactly the same. So you see, we can’t always find exact answers to problems quickly, but with science and engineering, you have the choice of knowledge to solve them as best as you can.