This is my first steemstem post, and it's on ammonia.
let's get started.
Flow of work
- Introduction
- Definition
- My experience with Ammonia
- Safety measures to ammonia exposure.
.
.
.
image source. Pixabay
Introduction
Biochemical hazard are seen almost everywhere in work places. Workers 👷 are always advised to make use of personal protective equipment (PPE).
When a substance is released either from a large area, it enters the environment. Such a release does not always lead to exposure.
Substance exposures exposure could occur only when in contact with it the substance which could be by breathing, eating, or drinking the substance, or by skin contact.
Ammonia exposure can cause harm as a result of many factors.
Some of these factors includes: the dose (how much), the duration (how long), and how you come in contact with it.
Ammonia is considered being a high health hazard to workers in places used as it is corrosive to the skin eyes and lungs.
Exposure to ammonia at lowdose causes irritation and chocking and at high dose it is immediately dangerous to life and health, being facilitated by diffusion.
Ammonia spills and releases pose a significant threat to workers from skin contact, inhalation, and fire and explosion tothe substance.
There are preventive measures put in places to aid workers who are exposed to ammonia at different levels.
Active words ammonia, hazard PPE.
Experience with ammonia
I have had some pretty cool experiences with ammonia.
When in senior high 2, I was made the chemistry lab prefect, probably because of my interest in the subject or because I preferred using the laboratory as my library, hence my regular presence at the lab.
On this particular day the lab teacher gave me the key to the lab to lock if she is not back before the hour of close. When it was time for me to go home, politely I asked the students reading and engaging conversations that its time to close.
Some left, but some seniors didn’t, they said, they would leave in their own time. I was both hungry and angry at such statement, I couldn’t do anything physical since they were more muscular and were my seniors in class. So I went into the lab inner chamber, brought out ammonia, poured out some into a beaker, wore a nose mask, then left the lab.
Within minutes, they all cam out coughing, I went back inside added water to the ammonia to dilute it, then discard it.
Hey! I know it was not fair of me, and now I can’t help but laugh at what gave me such ideas.
Oh! Yes ammonia can cause discomfort at even the lowest level when facilitated by diffusion and at higher level it can cause death.
AMMONIA
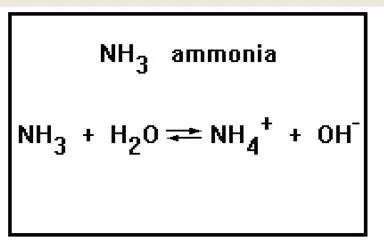
Ammonia can be defined as a chemical compound made up of one atom of nitrogen (N) element and three molecules of hydrogen (H).
It has the chemical formula of NH3.
It is made up of both by humans and by nature (Natural and Artificial production).
The amount of ammonia produced every year by humans is verymuch equal to the amount produced by nature every year.
However, ammonia thatis found at a concerned level to human’shealth, is likely produced either directly or indirectly by humans.
Large amounts of naturally occurring ammonia comes from animals, soil surface and also the human body.
Artificial processes of ammonia emission to the atmosphere includes fuel combustion and also in the treatment of sewage.
Nitrogen was first recognized as an important fertilizer in 1840, the first direct synthetic commercial process of ammonia was developed in Germany by fritz Haber and Carl Bosch in 1913.
At a point in life, almost everyone has smelled the penetrating odour of ammonia, as the active product of smelling salts; the smell can give a heart faint or a light headache.
Reaction of ammonia
NH3 (aq) + H2O (lq) <------> NH4+ (aq) + OH- (aq)
The water stand as a conjugate base while the products NH4 and OH stands as conjugate acid.
NH3-------ammonia
H2O-------water
NH4------ammonium ion
OH-------hydroxide.
The production of hydroxide ion when ammonia dissolves in water gives aqueous solutions of ammonia their characteristic alkaline properties. Thus it can act against microorganism and can be used to remove stains, hence used very well in cleaning household stuffs.
Uses of Ammonia
Ammonia as described earlier is a colourless alkaline gas and it is well recognized in usage around the homes as basis for household cleaning products.
It is also used in other sectors such as agriculture, industrial and commercial sectors make use of ammonia.
Ammonia usage is available in five (5) general recognized grades
• Fertilizer
• Refrigerant
• Federal
• Metallurgical
• Semiconductor
The refrigerator grade of ammonia usage is known to be 99.98 percent pure and is relatively considered free of water (anhydrous) and other impurities (max: 150ppm water, 3ppm oil, and 0.2ml/g non-condensable).
This form of ammonia is readily available and inexpensive and is capable of absorbing large amount of heat when it evaporates.
Ammonia in work places

ammonia uses in workplace
There are several workplaces commonly associated with potential ammonia exposure, and they are those that use ammonia as a refrigerant, such as ice rinks, cold storage plants, food and beverage manufacturing and processing facilities, and ice manufacturing plants.
Ammonia is flammable at concentrations of approximately 15% to 28% by volume in air. When ammonia is mixed with lubricating oils, its flammable concentration range increases and if released in an enclosed space with a source of ignition present. Fortunately, ammonia has a low odor threshold (20 ppm); hence most people will seek relief at much lower concentrations.
Hazard Hazard with ammonia
My second experience at the lab
Ammonia in the environment does not last very long, this is because it is recycled naturally.
There are many ways by which nature incorporates and transform ammonia.
In soil or water, plants and microorganisms rapidly take up ammonia.
After fertilizer containing ammonia is applied to soil, the amount of ammonia in that soil decreases to low levels in a few days. Ammonia will last about 1 week in air.
We have established the fact that ammonia at low concentration is offensive and people tend to take to their heels.
During my student work experience scheme (SIWES) which I did in the quality control unit of a pharmaceutical company. Since my supervisor was pregnant I was oriented on how to make use of the machines and the steps to carry out a quality assessment on drugs active pharmaceutical ingredients (API) and other analysis with supervision. One particular day, I was doing an experimental analysis that involved the use of concentrated ammonia, I never knew that the fuming chamber had developed fault, so with my protective personal equipment on (PPE), I opened the ammonia to transfer some content into a beaker for analysis in the fuming chamber.
In less than 5 minutes, everywhere was chocking and a bit foggy, I didn’t realize it until someone called my attention to it as people started leaving the lab coughing.
The ammonia was still opened.
.
.
.
Ammonia gas can be regarded as a compressed gas and a confined space explosion and toxicity hazard.
It is can decompose at a high temperature forming flammable hydrogen and nitrogen dioxide. This gas can lead to lungs injury, corrosive injury to the eyes and skin.
Levels of ammonia exposure

image source
Ammonia at 0.6-53ppm can be detected by people with severe respiratory tract irritation. Nose and throat irritation may be seen at concentration of 24ppm after 2-5 hours of exposure.
A 10 mins exposure to 30ppm of ammonia may be considered to be faintly irritating, while it is considered to be moderately irritating on exposure to 50ppm of ammonia.
Five minutes exposure to 72/ 134ppm of ammonia will cause irritation of the nose and throat for most people.
At 500ppm. Immediate and severe irritation of nose and throat occurs, and at 1500ppm brief exposure can cause pulmonary edema which may develop 1-24 hours after exposure.
This type of exposure is considered to be knock-out.
Reports from enviroMed detective services shows numerous cases of fatal ammonia exposure among workers concerned, but actual exposure levels have not been documented.
If victim survives, full recovery may occur depending on the injury to the respiratory tract and lungs.
Household and industrial cleaning solutions contains a in some cases.
The use of these products at home or work may lead to exposure to ammonia, this is because Household and industrial cleaning solutions are made by adding ammonia gas to water to form liquid ammonia.
Household ammonia cleaners contain lower levels of ammonia (between 5 and 10%) when compared to industrial cleaning solutions (up to 25%).
Farmers, livestock are also exposed to ammonia when working with or applying fertilizers containing ammonia to fields, and also from decaying manure.
Ammonia and your Health
Scientists make use of some test to protect the public from harmful effects of toxic chemicals and to find ways for treating persons who have been harmed. One way is to determine how the body absorbs, uses, and releases the chemical.
For some chemicals, animal testing may be necessary. Animal testing may also help identify health effects such as cancer or birth defects.
laboratory animals are so important of which without, scientists would lose a basic method for getting information needed to make wise decisions that protect public health. Scientists have the responsibility to treat research animals with care and compassion. Scientists must comply with strict animal care guidelines because laws today protect the welfare of research animals.
Inhalation of ammonia causes irritation of the eyes. Contact with concentrated ammonia lead to skin burn and at higher levels, immediate death 💀.
I have mistakenly drank ammonia one time during an experimental analysis at the lab. Though it was concentrated thank goodness. ☺. Instead of patiently getting another pipette handler, I used the old method, my mouth to suck out the solution putting in mind the pipette mark, but I got distracted and passed the mark then into my mouth.
I felt a little burn and soapy taste in my mouth. Washed my mouth with enough water.
Ammonia does not cause cancer ♋, according to EPA, the Department of Health and Human Services (DHHS), or the International Agency for Research on Cancer (IARC), ammonia has not been classified for carcinogenic effects.
- Some Beneficiary Effects of Ammonia**
There are many beneficial effects of ammonia, such as
when used as a smelling salt.
Certain ammonium salts have long been used in veterinary and human medicine.
Safety precautions
Engineering controls, proper training and the use of PPE are essential for workers exposed to ammonia.
Put a suitable respirator on immediate exposure to ammonia, and leave the area until the severity of the release is determined. In case of leaks, escape-type respiratory protective equipment should be available in the work area.
It is also important to never work alone with explosive chemicals.
Do not make use of incompatible materials such as oxidizing agents (Nitrogen oxide), Halogens (Chlorine, fluorine) and heavy metals (mercury, silver).
Do not open damage cylinder, it is also imperative for lab workers to slowly open chemical cylinder to prevent rapid decompression.
Conclusion
Ammonia occur in our everyday environments at different levels. Depending on the level of exposure it could have effect on the health of the individual exposed. Workers 👷 exposed daily should ensure proper PPE manners.
Thanks for reading
Follow me for more reads on
• Ammonia and my kids
• First Aid to ammonia inhalation
• Biological pathways of ammonia elimination from the body system
references
Toxic substance portal