Each one (Cycles) is just a sequence of events or steps that repeat themselves in the same order.
And that is exactly what is happening in some of the most commonly used devices that have ever been engineered. Like the heat pump in your home and the refrigerator that keeps your foods from spoiling.
Source: Wikimedia (Public domain - CCO licensed || Author: Pbmalloy (Einstein/Szilard with annotations by P. Brandon Malloy)
Hey man, welcome to this blog. This would be my first time writing a STEM post and well, I've got a lot of stuffs piled up. And here, we are talking about Cycles. So c’mon let's hit the road.
In engineering, we say that a system has undergone cycles if it returns to its initial state at the end of the process. So, the initial and final state should be the same. And cycles are important because they allow us to run through the same process again and again instead of limiting us to doing something only once.
With the right resources, we can keep running through Cycles until we get enough what we want, for instance, travelling in a car. We can also keep making cycles for as long as we need. Take for instance, trying to keep your food cold in a fridge at a constant temperature.
Brief history
The process that we use today is based on the work of these great scientists, Oliver Evans and Jacob Perkins. In 1805, Evans came up with a close vapor compression refrigeration cycle. But he never actually built a refrigerator. Then in 1830s, Perkins used Evans ideas and actually built something. Perkins built the first refrigerator and it was the first step towards the modern refrigerators that we use today.
So, how do these refrigeration cycles work?
Well, it is easy to understand if we take a look at the similar stuff that we already know a bit about - Heat engines.
Heat engines are machines or systems that convert heat into other forms of energy.
Basically, Heat Engine can do this by taking in heat at a higher temperature from let's say solar energy or furnace and then converting part of that heat to work. Engines normally release its wasted energy at a lower temperature maybe into its surrounding or water supply. Like many other things, heat engines operate on cycles. Cycles achieve some goal, like heating or cooling a room by circulating what is known as a working fluid through a series of operations. This working fluid will absorb and release energy for instance liquid to vapor and back again and continue to cycle it through the cycle as part of the system operations.
So let's look at this heat engine that uses water as its working fluid. It goes through four main stages. In the first stage, we add heat to our system by bringing in an energy source QH. The water will absorb this heat to a boiler which will cause it to become compressed steam. In stage two, that steam enters turbine, expands and causes the turbine shaft to turn which will give us an output of work, W-out which was converted from some of the heat energy in our fluid.
Remember, not all of the heat energy will convert to work. We can have excess heat which needs to be released from our system.
We get into stage three by condensing the steam in a condenser which releases the excess heat into an energy sink, Qr. For the fourth and final stage, all fluid needs to be re-pressurized. To make this happen, we send it through a pump which will need work as an input. We then send the re-pressurized back to the boiler at the beginning to start the process all over again. That is just one cycle and each one we do, we should have our output of work.
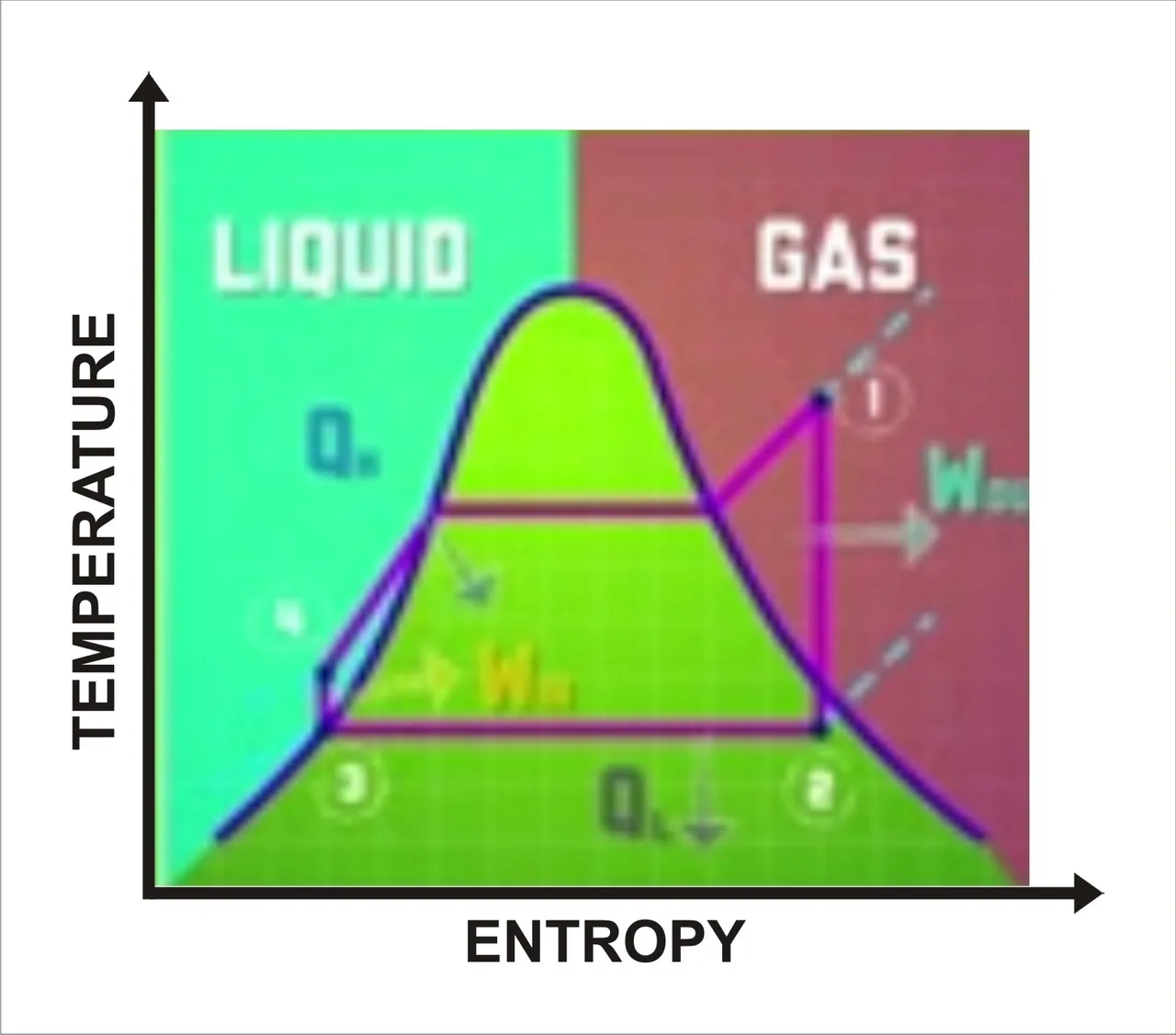
Heat engine fluid cycle. Sketched by me
If we look at the heat engine in a close system, when the title changes into kinetic energy, potential energy, and thermal energy through the cycle. So, as per the first law of thermodynamics, the changes in work and heat should equal themselves out. Now another way of looking at the cycle is by using a phase diagram. Phase diagrams compare different properties to show what state or fate a substance is in.
From this example, we compare entropy to the temperature of the heat engine fluid using the ranking cycle which is the ideal cycle for vapor power plants. If we take a look at the diagram, all material to the left of the curve is in liquid phase while all materials to the right is in gaseous state. Everything under the curve is a mixture of gas and liquid. The middle plot lets us easily see what state the fluid is in as it goes through each stage of the heat engine cycle.
Not only is the heat engine the great way to turn heat energy into work, but with a few changes, we can turn it into different types of system if we are trying to get work as an output. What if we try to get heat? Well, we do this every day when we try to heat or cool our homes. We use heat pump to add heat our system when we are chilling and a refrigerator to remove heat from the system when we want to keep things cool. In either case, we put work into the system rather than trying to get it out. What is interesting about refrigerators in heat pump is that since we are aiming for an output of heat, it is possible to get a hundred percent conversion of work which we know from the second law of thermodynamics. However, it is important to know that even though all of our work can't be converted into heat, it may not all be that heat that we want because we are still going to have two different temperature levels.
Refrigeration cycles
Now, with all that in mind, let's go back to refrigerator. If we are talking about the refrigerators in kitchens, the inside stays cool because of what is happening in its real interior wall. This is where our cycle will take place. Just like with the heat engine, we can break the cycle down into four different stages. Again, with the working fluid circulating through all the stages. In your kitchen refrigerator, this working fluid is hydroclorofloro carbon chemical which is referred to as FREON.
The first stage of the cycle is the evaporator which removes heat from the inside of the refrigerator. Turns out with the liquid fluid that colds in the inside of the refrigerator which is the result of last stage of the previous cycle. We will get to that. So this liquid is really cold but its boiling is also rally low. In fact, the liquid is just about the temperature of what is ready to boil. And as liquid change to gas, it absorbs heat. So when the liquid in the evaporator boils, it absorbs heat from the refrigerator and at the same time. But even though it absorbs heat, its temperature doesn't really change, only that heat energy is going to change the liquid into gas, not raising its temperature.
Stage two is the compressor. Its job is to raise the pressure of the gas, which also raises its temperature at the boiling point. Once the stage is complete, the gas is really hot. But because its boiling point increase too, its still about the boiling point trying to condense into a liquid which brings us to stage three; the condenser, which is basically the opposite of the evaporator in stage one.
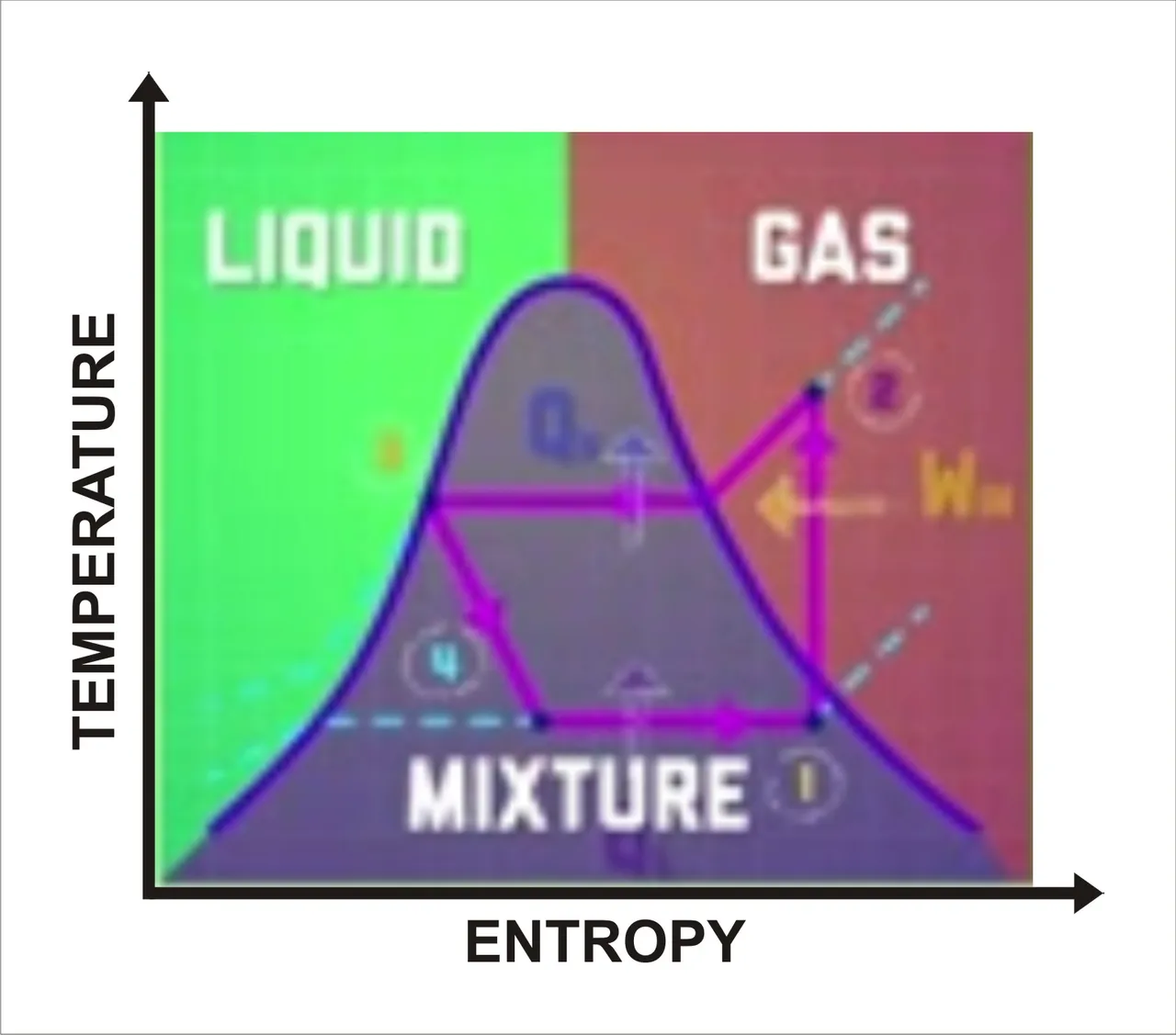
Refrigeration cycle. Sketched by me
In the condenser, the gas turns into a liquid, a process that releases heat. Since the refrigerator is now hot in here outside, it can flow from inside of the condenser to the surrounding air. But like in the evaporator, the temperature of the refrigerant stays constant in the condenser. Finally in stage four, the expansion value disturbs the liquid lower its pressure and thus lowering it temperature and its boiling point. It is the opposite of what happens in the compressor. You end up with a cold liquid refrigerant at a lower pressure ready to enter the evaporator and start the process all over again, absorbing more heat from the refrigerator as it boils.
So basically, the stuffs we have in refrigerator stay cool because we are taking heat out from the inside of your refrigerator. And we can see this more clearly if we take another look into this diagram, this time for refrigeration cycle. Similar to the phase diagram for the heat engine, we see a few more differences of more phase of the fluid at the different stages. The fluid spends more time in a gaseous state and less time as a liquid than a fluid did in heat engine.

Zeer pot. Source: Wikimedia (Public domain - CCO licensed || Author: Peter Rinker)
Zeer pot
Now full cycles are great but sometimes we need to have an incomplete cycle with a little outside help to get what we want. That is because sometimes, we are limited by our environment and what we have available. So for example, refrigerators often need electricity but that kind of power is not always available. So, with a little bit of problem solving, we can design one that doesn't need it. That was the idea behind ZEER POT. Zeer pot is a simple refrigerator made from one pot, settings are in order with a layer of wet sand in between them. It was made by a Nigerian man named Muhammad Bah Abba in 1990s. Similar device made its way back all the way to Egypt about 2500 BCE.
So, how does the Zeer pot work?
Well, as the moisture from the sand evaporates, it cools the inner pot by pouring out heat. It is a great way to have our refrigeration system in a hot climbing when we have very limited resources.
But why isn't the Zeer pot quite a cycle?
Without recapturing the evaporating water, we are going to need some outside work to make our sand wet again if we want to continue the cooling process. Sometimes we need to forgo the perfect cycle for the sake of practicality.
But all that being said, let's say we do have the resources for a refrigerator that can run on a cycle. How can we improve this process even more?
Well, one way is by using a renewable energy resource to fuel our system and produce the work that we need. Solar energy is a great example. Rather than taking electricity that was made from a typical source, we can use the solar panel to convert the sun rays into electricity by converting the electrons within the panel cells. The electricity that we get from this energy can replace the work that we need for the cycle, thus making the cycle itself more reusable which is great because the big goal for our engineers is to find ways from several processes even when something is already working. We can always improve our designs in one way or another. We can always take another step forward. The refrigerators that we make now are far better than the one Perkins made in 1800 and heat engines are getting more and more efficient as time goes on.