One of my uni essay :)
The fridge has a very particular purpose which is to maintain the temperature of the things inside it below those of the surroundings. In this sense, refrigeration is not a new concept. The oldest method to achieve this is to use natural ice. In 1806, Frederic Tudor, also known as the ice king, started a mass scale ice trade which transferred ice from North America the rest of the world including China and Australia. This brought refrigeration to mainstream use. To this day, many new types of fridge has been invented and implemented into daily lives.
The old fridge
In the past, a fridge would basically consist of an insulated box and ice in it. This kind of fridge relies on natural cooling processes to work. Techniques for refrigeration include nocturnal cooling and evaporative cooling.
Nocturnal cooling was perfected as early as 2500 B.C. A thin layer of water was poured onto trays, exposed to the cloudless night sky. The trays loose heat by radiation to the stratosphere. By early morning, water would have turned into ice. Interestingly, this only works when the sky is cloudless and the trays see the sky only, not the trees or buildings.
In evaporative cooling, water loses heat when evaporated to the surrounding air. If we think of a house as a huge fridge. Evaporative cooling can be practiced by placing wet straw mats on the windows. The water when evaporates to surrounding air absorbs its latent heat from the house and cool it. The mats also block and diffuse solar radiation on glass windows to reduce heat received.
The cooling methods relying on these natural processes depend on season and conditions which humans have little to no control over. Therefore, they are quite inconvenient. This leads to the invention of artificial refrigeration systems which are the fridges most people own today.
The modern fridge
Fridges nowadays can be controlled to reach a certain temperature and stay at that level for as long as needed. This allow the ability to store food and other substances safely for a long time.
To cool the air inside, fridges uses the thermodynamic application known as the reversed heat engine.
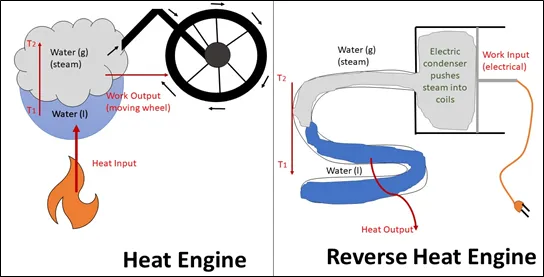
Figure 1: Heat engine and reverse heat engine
In a simple heat engine, inputted heat causes an increase in temperature of the working substance (water in Figure 1) allowing it to perform work. On the other hand, in a reverse heat engine, work is the input. The working substance is condensed and pushed into a heat-exchange coil. As the substance liquify and lower its temperature, it releases heat to the surrounding environment.
The substance used in the process is called the coolant, a special type of refrigerant. The coolant changes its phase from gas to liquid and back repeatedly during the refrigeration cycle. The next figure will show a simple example of the refrigeration cycle, which parts are inside or outside the fridge and how the coolant changes its phase.
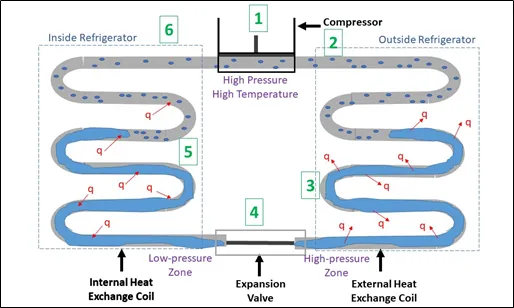
Figure 2: Steps in the refrigeration cycle
1. Outside the fridge, the compressor, powered by electricity does work on the coolant in gas phase, increasing its pressure. As the pressure is increased, so does the temperature (according to the ideal-gas law).
2. The heated gas enters the outside of the fridge.
3. Heat is transferred from the gas to the air of the room due to the temperature difference. The heat loss causes the coolant to change phase from gas to liquid. The work done by the compressor has been converted into heat transferred into the outside environment.
4. The expansion valve is placed in an insulated compartment of the fridge. The liquid coolant passes through the valve. It is now at a low pressure after expansion and is cooler than the air inside the fridge.
5. Because the liquid coolant has lower temperature than the air inside the fridge, it absorbs heat causing the temperature inside to reduce. As heat escape the coolant, its intermolecular attractions start to break allowing endothermic vaporization to occur.
6. When all the liquid coolant turns into gas, the cycle starts again.
This cycle does not run forever. To keep the temperature inside constant and stop the cycle when the desired temperature has been reached, a closed feedback control loop is used.

Figure 3: Refrigerator’s closed loop control
After turning on the fridge, a sensor (a thermostat in this case) is used to measure the temperature inside the compartment. This value of temperature is compared with the desired temperature. If it is higher, the compressor starts to work and begin the refrigeration cycle. When the desired temperature is reached, the compressor stops working. This saves electricity and ensure the system to work stably and efficiently.
References
1. Arora, R. (2012). Refrigeration and air conditioning. PHI Learning, pp.1-5.
2. Casiday, R. and Frey, R. (2007). Phase Changes and Refrigeration: Thermochemistry of Heat Engines. Washington University. [online] Available at: http://www.chemistry.wustl.edu/~edudev/LabTutorials/CourseTutorials/LabTutorials/Thermochem/Phase.pdf [Accessed 01 Feb. 2018].
3. Trott, A., Welch, T. and Hundy, G. (2008). Refrigeration and air-conditioning. Oxford: Butterworth Heinemann.
4. Lu, Y. (2009). The story of refrigerator and feedback control. Department of Electrical and Computer Engineering. [online] Available at: http://137.148.142.85/cactwiki/images/7/72/Refrigerator.pdf [Accessed 01 Feb. 2018].
5. Brain, M. and Elliot, S. (2006). How Refrigerators Work. [online] HowStuffWorks. Available at: https://home.howstuffworks.com/refrigerator.htm [Accessed 01 Feb. 2018].