Let's start by saying that quantum physics is a term we often hear but one that has such a vague idea that some people even use it to sell deceptive cures through the mind or supposedly fulfill your desires just by thinking about it.
Really what is quantum mechanics?
At the end of the nineteenth century physicists were very satisfied, Newton had described the movement of both planets and apples by simple equations and thanks to Maxwell had understood that electricity and magnetism were part of the same phenomenon, apparently, if you had enough data it was possible to predict or determine how any system would work until Max Planck arrived. He wondered why and how objects change color when heated, it happens because the energy they absorb is released in the form of light with different frequencies.
According to the classical mechanics at higher energy introduced the spectral radiance would increase exponentially tending to infinity, but the experiments showed that they did not happen so fast and that there was a limit to this failure of the classical theory, it is known as the ultraviolet catastrophe.
To solve this problem, just in 1900 Planck thought that instead of continuously measuring energy, he could measure it in indivisible quantities or packages that he called "Quantum", now everything fit, the minimum unit of magnitude of action, relationship between energy and time is now known as the Planck constant and any physical process can only be measured in whole multiples of this constant. (In the image: Quantum Hydrogen on Graphene; source)
For Planck this was a purely mathematical solution but shortly after Einstein recovered the concept and used it to explain and predict the photoelectric effect that earned him the Nobel Prize; the subatomic reality is quantum.
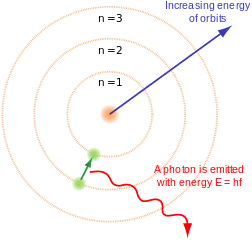
So much so that Niels Bohr used it to build his model of atom, in it, the electrons can be in certain orbits but never in an intermediate point, they are quantized and when they pass from one orbit to another, they emit a photon, the particle of the light.
In the image: Bohr atom model; Source
But ... is light a particle?
Scientists such as Christiaan Huygens, seeing phenomenon such as diffraction and refraction of light, saw that it behaved very similar to the waves they make in the water. They concluded that it was a wave.
Other scientists like Newton thought that understanding it as particles explained the matter better.
Before the quantum theory of Thomas Young had made an experiment between a light source and a dark wall, he placed a cardboard with 2 small slits, the image that was projected did not show 2 bars of light but several, this was a classic pattern of interference that would be obtained only if the light behaved like waves that were reinforced in some parts and canceled in others. This experiment is known as the "double slit experiment", it can be carried out by throwing one particle at a time.
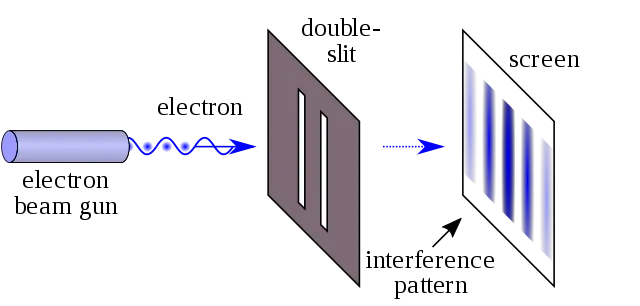
For example, a photon which could bounce off the barrier or go through one of the 2 slits and leave a mark when hitting the screen. If we did the experiment with objects, such as munitions, there would not be interference only two stripes of marks where the ammunition has hit. On the other hand, if we do it with subatomic particles, the result is the interference pattern that we already know. If we put a detector in the slits to know which of them has passed the photon, the interference pattern disappears and the light behaves like ammunition. It is as if only the observation changes the result of the experiment. This phenomenon is called the wave - particle duality of light.
The truth that both wave and particles are ideas of our everyday world that we try to use to explain the quantum world whose nature often goes against our intuition.
Quantum indeterminacy is also applied in the so-called "Hamlet" effect. The radioactive materials tend to decay until they cease to be if it is not observed, a radioactive atom is found in 2 states simultaneously, being and not being radioactive. Hence the mental experiment devised by Schrödinger, if the life of a cat depends on an atom in superposition of states the cat is alive and dead at the same time, it seems that it is until someone observes it. These strange results have had multiple interpretations, including one that says there are parallel universes, an interesting topic which I will try to explain later in another post.
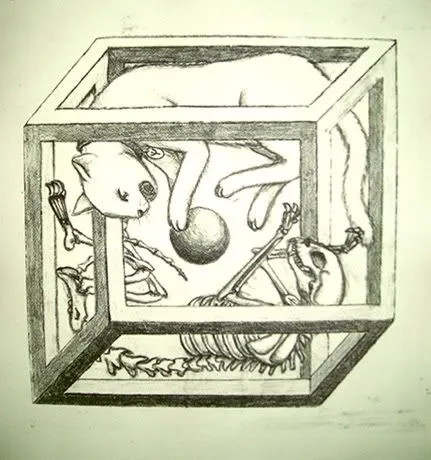
The paradox of Schrödinger's cat is an example of quantum superposition. Source
The images used in this article are Public Domain and can be reused according to your copyright.
if you liked the content of this publication, do not forget to support me @jlmol7
Additional Information.
https://www.livescience.com/32427-where-do-electrons-get-energy-to-spin-around-an-atoms-nucleus.html
References.
Ball, P. (2017). The strange link between the human mind and quantum physics. Bbc.com. Retrieved 8 April 2018, from http://www.bbc.com/earth/story/20170215-the-strange-link-between-the-human-mind-and-quantum-physics
Coolman, R. (2014). What Is Quantum Mechanics?. Live Science. Retrieved 8 April 2018, from https://www.livescience.com/33816-quantum-mechanics-explanation.html