From celebration to conversation to commiseration, most of us have no trouble rationalising the occasional drink. Whatever your brand of alcoholism, scientific curiosity, like most cognitive functions, generally takes a back seat when booze is involved. However, the cultured drinker among us might have pondered upon the well-known 'tears of wine' phenomenon. Well ponder no more...

If you drown your sorrows, not to worry: your Pinot Grigio cries too.
Surface Tension & The Marangoni Effect
We first need to understand surface tension, the skin-forming property of liquids and the reason light insects can sit atop water.

Surface tension beneficiaries: pondskaters.
In the bulk of a liquid, attractive forces of cohesion such as hydrogen bonding (which we've discussed previously) 'pull' evenly on a given molecule in all directions. At the liquid's surface, however, the outward attractive forces of adhesion are weaker than inward cohesion. Surface tension is a consequence of this force imbalance; a liquid minimises its surface area to lower the resultant internal pressure just like the taut surface of a filled balloon.
A liquid with a greater imbalance has a surface which exhibits more elastic behaviour and is said to have a greater surface tension.

What if we now introduced a surface tension gradient (adjacent areas of differing surface tensions)? Since a molecule in a liquid with a large surface tension attracts neighbouring molecules more strongly than a molecule in a low surface tension liquid, the liquid of low surface tension is 'pulled' towards the high tension liquid in a mixture - such mass transfer along a gradient is termed the Marangoni effect.
Volatility
To understand why wine cries, we also need to understand volatility, the tendency of a liquid to vaporise - or evaporate, at room temperature. A substance of higher volatility has a higher vapour pressure at equilibrium since it vaporises more readily (and molecules in the gas phase a apply proportional pressure on the surface of the liquid phase).
Putting It All Together
Adhesive forces allow a small amount of wine to 'stick' to the glass, resulting in a concave meniscus. At the top (thin end) of the meniscus, the greater surface area-to-volume ratio promotes faster evaporation than lower, thicker parts.
The evaporation-rate increase is greater for the ethanol content in the wine than the water. To justify this, weigh up the intermolecular forces in play: ethanol forms two hydrogen bonds per molecule, while water may form three, hence the former's lesser inward force imbalance and greater volatility. The tip must therefore soon become lower in ethanol concentration than the base of the meniscus.
Using the same consideration of force imbalance, we may conclude that water-rich liquid has a greater surface tension than water-sparse liquid.
In other words, a tension gradient must be present from base to tip, up which wine from below is pulled thanks to the Marangoni effect. This continues until the weight of the tea' formed becomes large enough to outweigh adhesion and the tear slides back down the glass before starting its journey all over again.
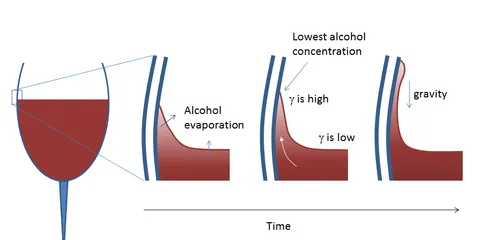
The mechanism and life-cycle of tears at the meniscus.
If you enjoyed this article and would like more, follow the everyday science blog for your daily dose of science.
References
The science of wineglass tears (or wine legs)
Why Does Wine Cry?
www.wt.kimiq.com
www.theatlantic.com
Tears of Wine and The Marangoni Effect by Brianne Costa