Here in the UK, snowfall is a relatively rare occurrence. When we finally do get our white Christmas, it wreaks havoc on the unprepared transport system, providing a convenient excuse for many 'not to make it in' to work or school.
Road gritting is a crucial tool in reducing the risk of accident on dangerous wintry roads, and the bane of opportunistic teenage truants worldwide.

Common sense surmises that grit must have some chemical effect on ice, but how does gritting return treacherous highways to commute-ready roads overnight?
What Is Road Gritting?
Road gritting is a process during which rock salt - specifically, halite, a mineral comprising sodium and chlorine - is applied to icy road surfaces.

In a basic sense, gritting 'melts the ice'. This can be misleading; some may infer that the grit reacts chemically with ice, but this is not the mechanism at play. Rather than changing the chemical makeup of the ice, gritting salt expands the liquid phase temperature region by lowering the freezing point of the road's surface.
Freezing Point
Most water molecules in the liquid state possess enough kinetic energy to overcome the crystalline structure favoured by intermolecular hydrogen bonding. Approaching 0 °(Celsius or Fahrenheit, not Kelvin!) the average water molecule has lesser energy and experiences a greater attractive force from its less spaced neighbours. It can no longer escape the structure, and octagonal crystals form - ice.

Hydrogen bonding in water/ice around the solid-liquid phase boundary.
Introduce grit and the bonding situation changes. The dissolved ions, positively charged sodium and negatively charged chloride, hamper hydrogen bonding. The partially polar water molecules are more strongly attracted to the ions than they are to each other, and the substance remains a liquid even at 0 °C.
The water will remain in the liquid phase so long as the ambient temperature provides its molecules with enough kinetic energy to overcome the effects of these weakened, ion-affected hydrogen bonds.
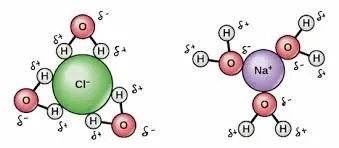
Salt ions interact with areas of charge within water molecules.
Gritted water typically has a freezing point between -4 ° and -10 °C, meaning even sub-zero road surfaces remain ice-free.

If you enjoyed this article and want more, follow the everyday science blog for your daily dose of science.
References: