
Deoxyribonucleic acid, or DNA as it is commonly known, is the molecule responsible for the passing of genetic information during cell division and the synthesis of proteins.
Chain Backbone
DNA is a polymer (repeated unit) consisting of two helical chains bonded to form a double helix shape. Each polymer is repeated and connected by phosphate groups. A single chain is of the form:
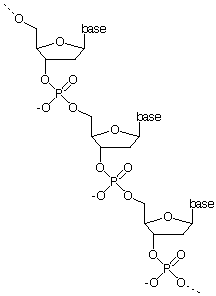
Bases
There are four different molecules that can be positioned as the 'base' attached to a chain: adenine, guanine, cytosine, and thymine. Two of these bases form a connection point between the two helical chains, and only complementary bases can form this connection - adenine and thymine, and guanine and cytosine, constitute pairs of complementary bases. The sequence of base pairings along the double helix is specific to a particular protein, and the transmission of the information required to code the correct sequence is crucial in new protein synthesis.
Why are DNA bases only compatible in this way?
Hydrogen Bonding
The answer to this question lies in the type of bonding responsible for the double helix structure. The structures of adenine and thymine, for example, are exactly matched and oriented to allow hydrogen bonding interactions - the strong attractive force between hydrogen and electronegative atoms such as oxygen, nitrogen and fluorine - whereas non-complimentary bases can interact only through weak intermolecular forces. This permits energetically favourable pairings between complimentary bases only.
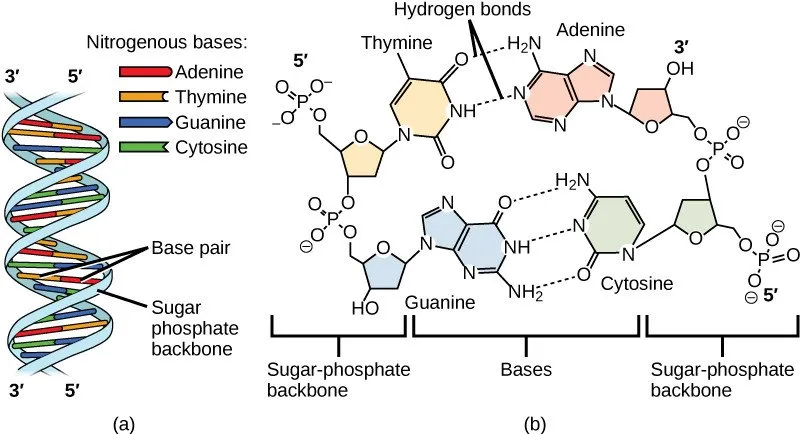
Hydrogen bonding between complimentary bases allows the connection of helical backbones
The mechanisms of natural phenomena can almost always be attributed to the bonding going on within, which makes scientific knowledge of the types and favouribility of chemical bonding essential in understanding biological processes, from cell division to photosynthesis.
If you enjoyed this article or are interested in all things science, follow my blog for daily doses of physics and chemistry, along with explanations for curious everyday phenomena.
References:
Chemistry 4th Edition by Housecroft and Constable